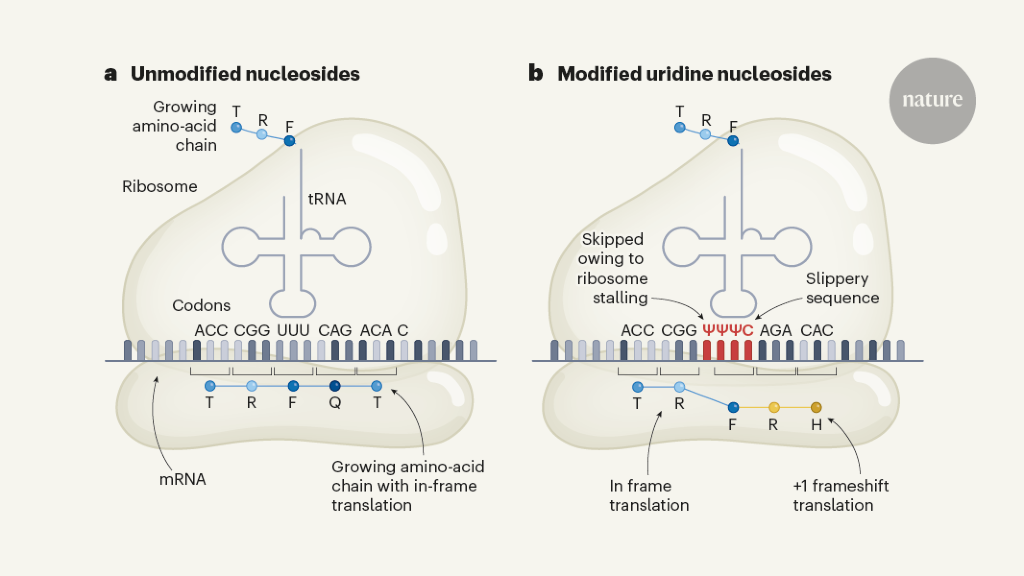
Advances made in messenger RNA technology led to two mRNA vaccines that target SARS-CoV-2 having an important role in the response to the COVID-19 pandemic. Rather than making the mRNA using cells, these vaccines use mRNA that is made in vitro in the laboratory and is known as in vitro transcribed (IVT) mRNA. Understanding the details of how IVT mRNA encodes protein during the process of translation (protein production mediated by the machinery of ribosomes and transfer RNAs) might contribute to future applications of this type of technology. Writing in Nature, Mulroney et al.1 reveal that the ribosome can stall at ‘slippery’ sequences along modified versions of mRNA (ones that include some nucleoside components that have been chemically modified); this might result in aberrant protein products. In the context of such nucleoside-modified mRNA vaccines and other non-vaccine therapeutic RNAs, this might lead to unintended consequences.
Competing Interests
D.B.W. notes several possible competing interests, which are managed by the Wistar General Council Office COI committee. These include consulting, BOD service, speaking, that can include in stock or monetary renumeration, and specific SRAs. Inovio Pharmaceuticals (BOD, consultant and SRA); AstraZeneca (speaker/consultant); Advaccine (consultant); Pfizer (speaker/advisory service); Geneos (consultant & SRA); Flagship (consultant); Sumitomo Pharma (consultant); plus possibly others that are managed by Wistar COI Committee. D.B.W. is a member of the International Society for Vaccines, AAI, ASGCT, AAAS, among other scientific societies. He also serves on NIH and NCI study sections and similar activities for other agencies.